I know, this is plunging in deep, but semantics is something that has come to bug me and haunt me. Presenting ideas to international collaborators from academia and industry - orally or in writing - I want to be understood correctly. When we exchange thoughts and juggle concepts with like-minded researchers (often on the other side of the world) there is a lot of potential for greatness. Isn't it a great feeling to go to bed pondering about a problem and to wake up to find the answer in your inbox, neatly written out by your friends in Japan, Australia or America?We all speak English, we are wrecking our brains looking for answers to the same questions, we worry about the same problems. It keeps amazing me!
But then, who of us has not seen two brilliant professors arguing across a lecture room, not listening to each other, and mixing everything up. Hopefully, they will realise quickly that they mean the same thing! They are just calling it something different.
In the stress corrosion cracking community there are a number of terms/phrases/expressions which I find keep causing confusion. I may have to add to the list later, but here is the ones that I feel keep coming up.
Stress Corrosion Cracking (SCC) or Hydrogen Embrittlement (HE)
It has been suggested that both expressions refer to the same phenomenon. Both can refer to a mode of failure, that would not occur without the coaction of mechanical forces and changes in the local chemistry. However, HE can definitely occur without SCC - in hydrogen charged samples with no corrosion to be seen. It is more difficult the other way around. H gas tends to be released in corrosion/oxidation of metals in aqueous environments
M(2+) + 2 H2O ⇌ M(OH)2 + 2 H(+)
M(3+) + 3 H2O ⇌ M(OH)3 + 3 H(+).
This means that there will be H gas is almost always present in corroding areas and there is a possibility that HE is responsible for cracking.
There are many other good theories competing with the one of HE though. The corrosion products themselves form a brittle phase prone to cracking. The adhesion between metals and oxides is another weak point.
It was even suggested that corrosion causes vacancy injection and local enhancement of the metal plasticity, thus facilitating slip leading to exposure of fresh metal and crack growth.
The two expressions, SCC and HE, are definitely not synonyms for one and the same mechanism and it remains to be determined if SCC is aided by HE.
Ni-enrichment or Cr(Fe)-depletion?
I think I may be changing my mind on this. :)
In an Fe-Cr-Ni alloy surely both expressions mean exactly the same thing. If you talk about one, the other is automatically implied. However, there is a subtext. Calling it one thing or another, we suggest, which elements we believe are moving and which ones are staying behind. The tendency seems to be, that people working on Fe-base steels talk about Ni-enrichment, while people working on Ni-base alloys prefer Cr-depletion.
I suppose it is not that surprising that I am changing my preference having moved from one materials to the other. As both expressions essentially mean the same, you may say this is not really an issue. However, I have confused myself and others frequently with this. Maybe it is necessary to say both every time.
Testing and Exposure
This is something that I believe is born, because people (i.e. me) talk about things they don't understand. I work on materials characterisation. I don't expose things. I don't test things. Anyway. Testing - so I was told - refers to SCC testing (or tensile testing, hardness testing, etc.) where there is a ASTM standard defining the TEST procedure. If you're just boiling a piece of metal in an autoclave for a few month, to test - erm... find out - what sort of oxides are forming, that is an exposure.
Internal Oxidation
My suggestion is: Let's never say these words again! The problem is, we have no idea what this means anymore. Traditionally metallurgists speak of Internal Oxidation, if less noble minor alloying elements for oxides inside a more noble matrix at high temperature (>500 C) . These oxides can be intergranular or transgranular. They often form disconnected precipitates throughout the entire bulk of the material.
When Scott and LeCalver saw discontinuous Fe- and Cr- oxides at grain boundaries in Ni-base alloys exposed to water at PWR operating temperatures (~300 C), which are MUCH lower, they concluded Internal Oxidation is contributing to SCC.
Other groups have later found discontinuous oxides at grain boundaries in stainless steels. They saw the resemblence with Scott and LeCalver's results and Internal Oxidation was mentioned in connection with steels as well.
This is problematic, because the original definition does not apply in a steel. The oxides, continuous or not, are Fe- and Cr-oxides. These are the main alloying elements, not some minor additions. The reason for the preferential oxidation of discrete areas are higher defect densities and fast diffusion paths. There is no difference chemically.
Except.
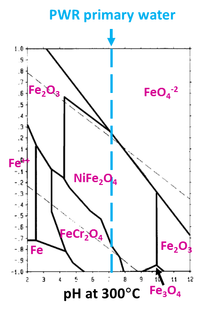
In some cases there are Cr-depleted/Ni-enriched areas at the oxidation front. It has been argued that locally the definition of Internal Oxidation still makes sense. And it does sort of. We can probably agree that we cannot talk about SCC austenitic steels and Ni alloys without studying the Fe-Cr-Ni Pourbaix diagram.
I think we have thoroughly confused ourselves and we need to stop talking about Internal Oxidation in
connection with SCC. I think it makes more sense to talk about discrete intergranular oxides at least until we have a better idea.
But then, who of us has not seen two brilliant professors arguing across a lecture room, not listening to each other, and mixing everything up. Hopefully, they will realise quickly that they mean the same thing! They are just calling it something different.
In the stress corrosion cracking community there are a number of terms/phrases/expressions which I find keep causing confusion. I may have to add to the list later, but here is the ones that I feel keep coming up.
Stress Corrosion Cracking (SCC) or Hydrogen Embrittlement (HE)
It has been suggested that both expressions refer to the same phenomenon. Both can refer to a mode of failure, that would not occur without the coaction of mechanical forces and changes in the local chemistry. However, HE can definitely occur without SCC - in hydrogen charged samples with no corrosion to be seen. It is more difficult the other way around. H gas tends to be released in corrosion/oxidation of metals in aqueous environments
M(2+) + 2 H2O ⇌ M(OH)2 + 2 H(+)
M(3+) + 3 H2O ⇌ M(OH)3 + 3 H(+).
This means that there will be H gas is almost always present in corroding areas and there is a possibility that HE is responsible for cracking.
There are many other good theories competing with the one of HE though. The corrosion products themselves form a brittle phase prone to cracking. The adhesion between metals and oxides is another weak point.
It was even suggested that corrosion causes vacancy injection and local enhancement of the metal plasticity, thus facilitating slip leading to exposure of fresh metal and crack growth.
The two expressions, SCC and HE, are definitely not synonyms for one and the same mechanism and it remains to be determined if SCC is aided by HE.
Ni-enrichment or Cr(Fe)-depletion?
I think I may be changing my mind on this. :)
In an Fe-Cr-Ni alloy surely both expressions mean exactly the same thing. If you talk about one, the other is automatically implied. However, there is a subtext. Calling it one thing or another, we suggest, which elements we believe are moving and which ones are staying behind. The tendency seems to be, that people working on Fe-base steels talk about Ni-enrichment, while people working on Ni-base alloys prefer Cr-depletion.
I suppose it is not that surprising that I am changing my preference having moved from one materials to the other. As both expressions essentially mean the same, you may say this is not really an issue. However, I have confused myself and others frequently with this. Maybe it is necessary to say both every time.
Testing and Exposure
This is something that I believe is born, because people (i.e. me) talk about things they don't understand. I work on materials characterisation. I don't expose things. I don't test things. Anyway. Testing - so I was told - refers to SCC testing (or tensile testing, hardness testing, etc.) where there is a ASTM standard defining the TEST procedure. If you're just boiling a piece of metal in an autoclave for a few month, to test - erm... find out - what sort of oxides are forming, that is an exposure.
Internal Oxidation
My suggestion is: Let's never say these words again! The problem is, we have no idea what this means anymore. Traditionally metallurgists speak of Internal Oxidation, if less noble minor alloying elements for oxides inside a more noble matrix at high temperature (>500 C) . These oxides can be intergranular or transgranular. They often form disconnected precipitates throughout the entire bulk of the material.
When Scott and LeCalver saw discontinuous Fe- and Cr- oxides at grain boundaries in Ni-base alloys exposed to water at PWR operating temperatures (~300 C), which are MUCH lower, they concluded Internal Oxidation is contributing to SCC.
Other groups have later found discontinuous oxides at grain boundaries in stainless steels. They saw the resemblence with Scott and LeCalver's results and Internal Oxidation was mentioned in connection with steels as well.
This is problematic, because the original definition does not apply in a steel. The oxides, continuous or not, are Fe- and Cr-oxides. These are the main alloying elements, not some minor additions. The reason for the preferential oxidation of discrete areas are higher defect densities and fast diffusion paths. There is no difference chemically.
Except.
In some cases there are Cr-depleted/Ni-enriched areas at the oxidation front. It has been argued that locally the definition of Internal Oxidation still makes sense. And it does sort of. We can probably agree that we cannot talk about SCC austenitic steels and Ni alloys without studying the Fe-Cr-Ni Pourbaix diagram.
I think we have thoroughly confused ourselves and we need to stop talking about Internal Oxidation in
connection with SCC. I think it makes more sense to talk about discrete intergranular oxides at least until we have a better idea.
 RSS Feed
RSS Feed